Share Article
How to Export Public Key in Internet Explorer – FDA ESG
Read our guide on how to export public key in internet explorer

Export Public Key
Open Internet Explorer. Click on the gear icon in the top right-hand corner.
Click Internet Options.
Note: Depending on your Internet Explorer version you may also find this in Tools > Internet Options:
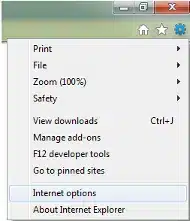
Click the Content tab. Click Certificates:
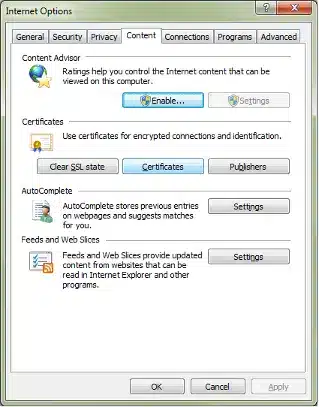
Highlight your Client Digital Certificate you intend to use for FDA submissions.
Click Export:
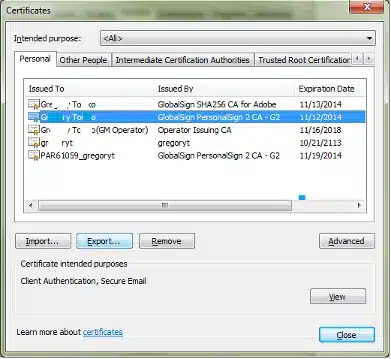
Note: If you are renewing a certificate, you can differentiate the new certificate from the old one by highlighting a certificate and clicking View. This will bring up the certificate details. The Valid From date will help indicate which certificate was issued most recently.
The Certificate Export Wizard will start. Click Next:
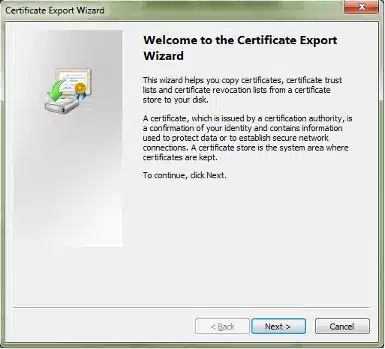
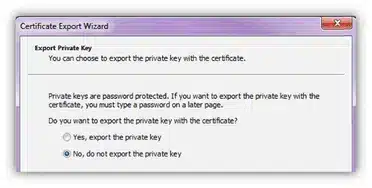
Select the No, Do Not Export the Private Key option. Click Next:
Select the Export File Format options listed below:
Cryptographic Message Syntax Standard – PKCS#7 Certificates (.P7B)
Include all certificates in the certificate path if possible.
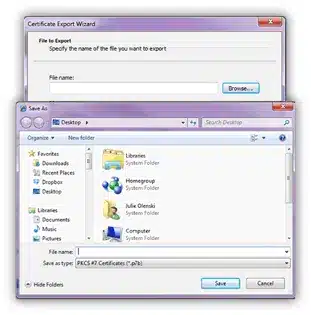
On the Certificate Export Wizard window click the Next button to continue with the export.
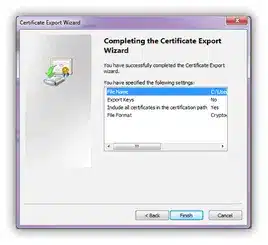
Click the Finish button on the following screen to complete the export:
Export was successful. Click OK:

You have now successfully exported your Public key.
The next step is to set up a test account; you’ll upload your public key during this process. If this is a renewal or reissue on an existing account, skip ahead to updating your public key.
Setting up a test account
You will receive a reply to your request in Step 1 from the FDA containing a temporary UserID and Password for your WebTrader test account. Once you receive this email, you are ready to set up the test account. Be sure to have the following items available during this process:
- Company and Contact Information
- Your Public Key (.p7b) that you exported
Click on the Login link in the e-mail provided by the FDA.
Enter your User ID and Password.
Click Log in:
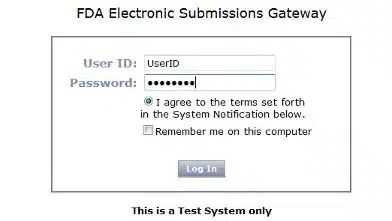
This will begin the WebTrader Registration Wizard.
Click Next:
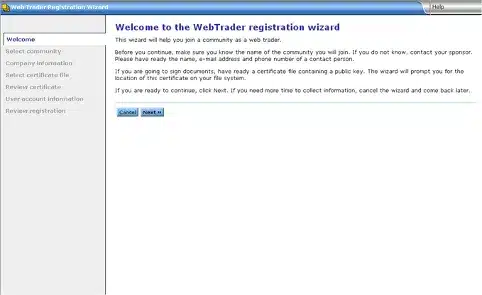
Choose FDATST from the drop-down menu on the Pick a community page:
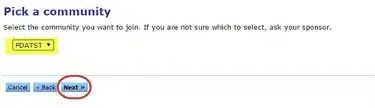
Enter your Company Name.
Click Next:
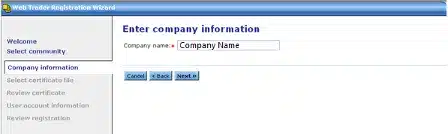
Click Browse and select your Public Key that you exported earlier..
Click Next:
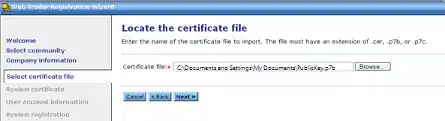
Review your certificate details on the next screen for accuracy.
Click Next.
Create a User ID and password.
Enter your contact information.
Click Next.
Note: This new User ID and password will be used for future logins:
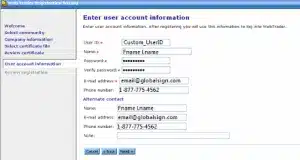
Review your order summary for accuracy and press Finish when you are ready to complete the process. You will be returned to the Login page.
Typically on the next business day, the FDA will send an e-mail notification to the address entered during Test Account Registration indicating approval to send a test submission. You cannot complete a test submission until you receive this approval from the FDA. Once you receive this e-mail, continue on to the next step.
Complete a Test Submission – FDA ESG
Navigate to https://esgtest.fda.gov/ to launch the FDA ESG Web Trader:
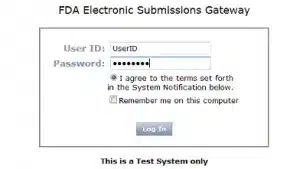
Enter your User ID and Password.
Agree to the terms.
Press Login.
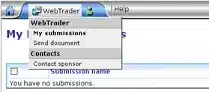
From the Web Trader menu on the top left, select Send document:
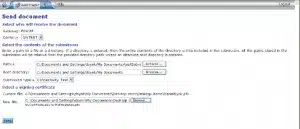
On the Send Document page, do the following:
Select an FDA Center from the Center drop-down menu
Click Browse in the Path field.
Select the file or folder you intend to use for your test submission.
The Root Directory field will populate automatically.
Select a Submission Type from the drop-down menu.
Click Browse to select your signing certificate. This is the .pfx you downloaded from GlobalSign.
Click Send:
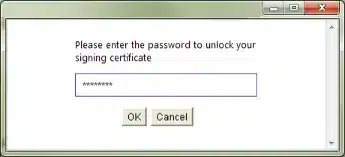
Enter the password for your signing certificate (private key). Press OK:
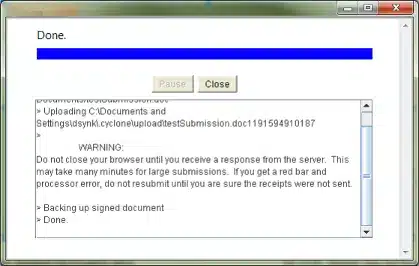
The Upload Progress dialog box will appear.
Once it says Done, your submission is complete:
For more detailed information about sending test submissions to the FDA, please see section 4.3 of the FDA’s ESG User Guide.
Steps for the FDA ESG Account:
- Email the public key (.p7b) to ESGHelpDesk@fda.hhs.gov, providing the following information:
- Primary account holder’s name
- +Electronic Submissions Gateway Account name
- A confirmation email from ESGHelpDesk@fda.hhs.gov will be received, notifying the account holder that the new public key has been uploaded. The user may now send submissions via the Electronic Submissions Gateway using the newly exported private key (.pfx).
Submit Your Technical Queries Here for Expert Assistance!
We will contact you as soon as possible.
Please enter your details below.

